valence electrons in aluminum|how many valence electrons does c have : Tuguegarao Mar 23, 2023 Our sweepstakes management company takes on legal compliance, official rules, purchasing & shipping prizes, tax forms, and everything else involved with your promotion. You know your business better than anyone else. We know sweepstakes better than anyone else. Together, we can create a robust plan that will allow you to acquire new .
valence electrons in aluminum,Mar 23, 2023 The valence of an atom is the number of electrons with which it will bond or . There are two ways to find the number of valence electrons in Aluminum (Al). The first is to use the Periodic Table to figure out how many electrons Aluminum has in its valence . Example \(\PageIndex{1}\): Number of Valence Electrons. How many valence electrons are in one atom of each element? sulfur; helium; potassium; . Aluminum (also called Aluminium) is the third most abundant element in the earth's crust. It is commonly used in the household as aluminum foil, in crafts such .
valence electrons in aluminum 6. An aluminum atom has three valence electrons. Do you think it will lose three electrons or gain five electrons to obtain an octet in its outermost electron shell? .An atom's valence electrons are the electrons in its outermost shell. In the chlorine model below, the valence electrons are shown in red . A Bohr model of a chlorine atom . Al is metal used as a nonferrous metal. Its compound is mostly present in the form of rocks, vegetation, and animals. The atomic weight of the aluminum is .
Explanation: The electron configuration for Aluminum is. 1s22s22p63s23p1. The first 10 electrons are in the filled first and second shells. This leaves only three .
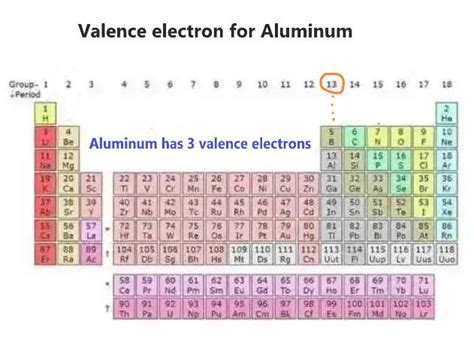
Jul 15, 2017. The number of valance electrons is 3. Explanation: The electron configuration for Aluminum is. 1s22s22p63s23p1. The first 10 electrons are in the filled first and second shells. This leaves only three electrons in the third shell 3s23p1. 13 −10 = 3. so there are only 3 valance electrons.Element Aluminium (Al), Group 13, Atomic Number 13, p-block, Mass 26.982. Sources, facts, uses, scarcity (SRI), podcasts, alchemical symbols, videos and images. . Members of a group typically have similar properties and electron configurations in their outer shell. Period A horizontal row in the periodic table. The atomic number of each .Answer: Aluminum has 3 valence electrons. Explanation: Valence electrons are the electrons present in the outermost energy level (shell) of an atom. Aluminum (Al), with an atomic number of 13, has an electron configuration of 1s 2 2s 2 2p 6 3s 2 3p 1. In this configuration, the outermost shell is the third shell (3s 2 3p 1 ), which contains 3 .
As a gas or vapor, the halogens all had a pungent odor. After the development of quantum mechanics, it was shown that the halogens all had seven valence electrons, supporting their original placement into the same group on Mendeleev's periodic table. Figure 11.1.1 11.1. 1: Periodic table by Dmitri Mendeleev, 1871. Figure 2.4.2 2.4. 2: Electron diagram for magnesium. The electron arrangement also shows the number of valence electrons which is two for magnesium because there are two electrons in the n = 3 n = 3 energy level which is the highest occupied energy level for magnesium. This corresponds to the 2+ 2 + charge formed . Valence electrons. Valence electrons are the electrons in the outermost shell, or energy level, of an atom. For example, oxygen has six valence electrons, two in the 2s .Valence electrons are responsible for the reactivity of an element. They determine how "willing" the elements are to bond with each other to form new compounds. If the valence shell of an element is full, such as with a noble gas, then the element does not want to gain or lose an electron. For example, alkali metals, which all have a valency of .Overview Electron configuration. The electrons that determine valence – how an atom reacts chemically – are those with the highest energy.. For a main-group element, the valence electrons are defined as those electrons residing in the electronic shell of highest principal quantum number n. Thus, the number of valence electrons that it may have .

Therefore, the valence electrons of aluminum are three. The last shell of aluminum has three unpaired electrons, so the valency of aluminum is 3. The last electron of aluminum enters the p-orbital. Therefore, it’s a p-block element. To know these properties of aluminum one must know the number of electrons and protons of aluminum. . Aluminum Valence Electrons: Aluminum is the chemical element denoted with a symbol (Al) and with an atomic number 13. Aluminum is in silvery-white color metal. Al is metal used as a nonferrous metal. Its compound is mostly present in the form of rocks, vegetation, and animals. The atomic weight of the aluminum is 26.9815. Aluminum has 3 valence electrons because there are 3 electrons present in the outermost shell of the Aluminum (Al) atom. Now let’s see how you can easily find the valence electrons of an Aluminum atom (Al). If you don’t want to read the texts, then you can also watch this video.
Boron and aluminum, for example, they can form stable molecules where the boron or the aluminum only have six valence electrons, not eight. And there are exceptions in the .
When forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. For example, fluorine has seven valence electrons, so it is most .
valence electrons in aluminum how many valence electrons does c haveHow many electrons does aluminum-27 have in its valence electron shell? This question is asking us to determine the number of electrons found in the valence electron shell of the aluminum-27 isotope. First of all, the valence electron shell is the outermost electron shell of an atom. And the electrons found in this shell are called valence .Flexi Says: Aluminum is in group 3A and has 3 valence electrons. Discuss further with Flexi. Ask your own question! Aluminum is in group 3A and has 3 valence electrons.
Explanation: Aluminum is the third element in the third row of the periodic table. The outermost orbitals to be filled are 3s and 3p. The 3s orbital fills before the 3p orbitals. So, the electron configuration is [N e]3s23p1. Just by looking at the electron configuration, you see that there are three valence electrons.

A sheet of aluminum foil and a copper wire are both places where you can see metallic bonding in action. . (since metals are located on the left side of the periodic table and do not have many electrons in their valence shells). The theory must also account for all of a metal's unique chemical and physical properties.how many valence electrons does c haveAluminium has three extra electrons that fluorine atoms can easily use. Since aluminium has three valence electrons, three fluorine atoms can bond. AlF 3 is the chemical formula. Aluminium trichloride. Chlorine (Cl) can also form a bond with aluminium (Al). Aluminium has three extra electrons that can easily be used by chlorine atoms to bond.
valence electrons in aluminum|how many valence electrons does c have
PH0 · valence electrons chart
PH1 · transition metals valence electrons list
PH2 · number of valence electrons list
PH3 · number of valence electrons fe
PH4 · how to find valence electrons
PH5 · how many valence electrons does silver have
PH6 · how many valence electrons does c have
PH7 · aluminum valence shell
PH8 · Iba pa